Abstract
Background: Myelofibrosis (MF) is a clonal stem cell neoplasm characterized by excess fibrous tissue formation in the bone marrow. Allogeneic hematopoietic cell transplantation (HCT) is the only curative treatment for MF. Patients with MF often have compensatory splenic extramedullary hematopoiesis leading to splenomegaly, which is associated with delayed engraftment and decreased survival post-HCT. Several Janus kinase (JAK) inhibitors are approved to treat splenomegaly and systemic symptoms in MF patients. However, JAK inhibitor therapy does not always lead to complete resolution of splenomegaly. Therefore, splenic irradiation (RT) prior to HCT may benefit patients with persistent splenomegaly, yet research investigating outcomes of this therapeutic approach is limited.
Methods: We conducted a single-center, retrospective cohort study of MF patients to characterize the feasibility of splenic RT and assess engraftment rates and post-transplant outcomes with this approach. Forty-eight MF patients who underwent HCT at Massachusetts General Hospital between 2011 to 2021 were identified using an institutional transplant database. Patient disease- and transplant-related characteristics were summarized according to splenic RT within 3 months prior to transplant.
Results:
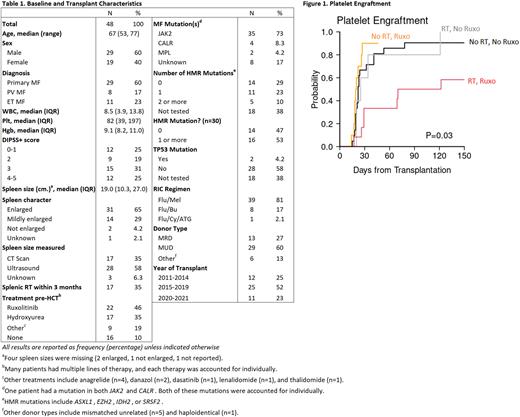
Baseline disease- and transplant-related characteristics are displayed in Table 1. Among all patients, twenty-nine (60%) were male, and the median age was 67 years (range, 53-77). All patients received reduced intensity conditioning, with a majority (81%) receiving fludarabine with melphalan. Seventy-three percent of patients had a JAK2 mutation and 46% received ruxolitinib (RUX) prior to HCT. A total of 17 (35%) patients received splenic RT prior to transplant. Splenic RT ranged from 2 daily doses of 1 Gy to 5 daily doses of 1.5 Gy, with total radiation ranging from 2 Gy to 7.5 Gy. Splenic RT was administered at a median of 13 days prior to transplant (range, 7-69 days). All splenic RT patients had splenomegaly prior to transplant, and 11 (65%) were treated with RUX prior to HCT. Patients receiving splenic RT had significantly larger spleen sizes prior to transplant (23.1 v. 17.3 cm, p<0.01), and were more likely to have at least one high molecular risk (HMR) mutation than patients in the no RT group (83% v. 33%, p=0.01).
For the entire cohort, the median days to neutrophil engraftment and platelet engraftment was 14 and 23, respectively. Among splenic RT patients, there was one case of primary graft failure and one case of secondary graft failure. The median days to neutrophil engraftment was 15 (IQR, 13-19) for the RT group and 13 (IQR, 12-14) for the no RT group (p=0.07). The median days to platelet engraftment was 32 (IQR, 25-108) for the RT group and 22 (IQR, 18-25) for the no RT group (p<0.01). Three splenic RT patients (18%) died early without platelet engraftment. When RUX was accounted, patients with RT and RUX had a substantially delayed platelet engraftment compared to no RT and/or no RUX (69.5 days vs 19.5-22.5 days, p=0.03) [Figure 1]. For the entire cohort, the 3-year overall survival (OS) and progression-free survival (PFS) was 67% (95%CI: 50%-79%) and 61% (95%CI: 45%-74%), respectively. The cumulative incidence of non-relapse mortality (NRM) and relapse was 26% (95%CI: 13%-40%) and 13% (95%CI: 5%-24%), respectively. Despite delayed platelet engraftment, survival outcomes were not different between RT and no RT, regardless of pre-HCT RUX exposure.
Conclusion: Splenic RT administration prior to HCT appears feasible and associated with acceptable engraftment and clinical outcomes for MF patients with splenomegaly at the time of transplant. The persistence of massive splenomegaly at transplant following pre-transplant RUX is likely indicative of higher risk disease and remains associated with longer time to platelet engraftment, despite the addition of splenic RT. Larger prospective studies are required to fully assess the clinical impact of splenic RT in the transplant setting, especially in combination with other therapeutic strategies.
Disclosures
Patel:Suffolk University Dosimetry Program: Honoraria; Teladoc Health: Other: Expert Witness, payment for second opinion on hematologic radiation; Tufts Medical Center: Honoraria. Spitzer:Syneos Health: Membership on an entity's Board of Directors or advisory committees, Other: Data monitoring and adjudication committees; Ossium Health: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees, Other: Data monitoring and adjudication committees; Qihan Biotech: Membership on an entity's Board of Directors or advisory committees. Chen:Gamida Cell: Consultancy; Novartis: Consultancy; Equillium: Consultancy; Celularity: Consultancy; Incyte: Consultancy; Jasper: Consultancy; Actimium: Consultancy. Defilipp:Omeros: Consultancy; Kadmon: Consultancy; Syndax Pharmaceuticals: Consultancy; Taiho Oncology: Research Funding; Regimmune: Research Funding; Incyte: Consultancy, Research Funding. Hobbs:Keros: Other: Advisor or review panel participant; Bristol Myers Squibb Co./Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Incyte: Other: Advisor or review panel participant; PI, Research Funding; Pharmaxis: Other: Advisor or review panel participant; Constellation: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; PI, Research Funding; Pfizer: Other: Advisor or review panel participant; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Abbvie Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Bayer: Research Funding; Merck: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.